The study of fundamental interaction between chromophores and environment (liquid solutions and solid films and bulks) are the basic knowledge to operate in the filed of doped and functionalized materials. In particular the optical properties of the dye molecules in a glass matrix change, with respect to those of liquid solutions, as an effect of the physical and/or chemical interaction with the silica rigid cage. In some cases this leads to advantages in terms of increased radiative efficiency, improved long-term photostability, reduction of the molecular dimerization.
As an example we reported the case of DODCI dye molecule. The DODCI molecule can be found in liquid solution in two isomeric conformation with separate radiative de-excitation trough fluorescence both to N- and P-isomer (respectively at lem» 605nm and lem» 650nm in methanol). As a result of the different nonradiative transition rates, the populations of the electronic levels and the photoemission spectra depend strongly on the average intensity and the time duration of the exciting light.
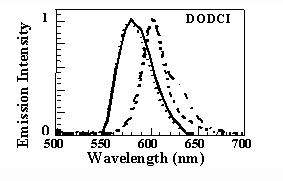
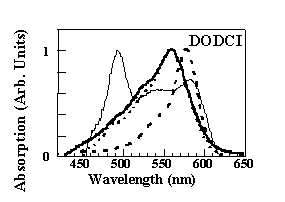 Dynamics of photoisomerization from the initial N-isomer to the P-isomer conformation is described satisfactorily with a three valley energy diagram involving an intermediate state. The physical constraint imposed on the molecules by the dimensions of the silica cage after densification affects the electronic structure and increases the energy of the N-isomer excited state. This results in a blu-shift of the absorption and emission bands of about 20 nm with respect to the bands in not-densified samples (thin line) which coincide with those in liquid solution (10-6M solution in methanol, dashed line). It should be noted, however, that in the nondensified samples under consideration, the absorption spectrum differs from that of methanol because of a new absorption band at approximately 490 nm. This new structure was attributed to the interaction between the silanol groups of the silica cage and a fraction of the dye molecules that are trapped in the pores of the glass matrix. Moreover the emission spectra show that, differently from the liquid solution where the P- to N-isomer emission intensity grows with the average intensity of the exciting light (300 mW/cm2, dashed line; 50 W/cm2, dash-dotted line), the photoisomerization process is strongly quenched (full line). This is likely to occur because of the rigidity of the silica cage since the isomerization in DODCI is very sensitive to the environment viscosity and requires an increase of the molecular length. The presence of the silica rigid cage affects also the relaxation processes. The time decay of theN-isomer photoluminescence
Dynamics of photoisomerization from the initial N-isomer to the P-isomer conformation is described satisfactorily with a three valley energy diagram involving an intermediate state. The physical constraint imposed on the molecules by the dimensions of the silica cage after densification affects the electronic structure and increases the energy of the N-isomer excited state. This results in a blu-shift of the absorption and emission bands of about 20 nm with respect to the bands in not-densified samples (thin line) which coincide with those in liquid solution (10-6M solution in methanol, dashed line). It should be noted, however, that in the nondensified samples under consideration, the absorption spectrum differs from that of methanol because of a new absorption band at approximately 490 nm. This new structure was attributed to the interaction between the silanol groups of the silica cage and a fraction of the dye molecules that are trapped in the pores of the glass matrix. Moreover the emission spectra show that, differently from the liquid solution where the P- to N-isomer emission intensity grows with the average intensity of the exciting light (300 mW/cm2, dashed line; 50 W/cm2, dash-dotted line), the photoisomerization process is strongly quenched (full line). This is likely to occur because of the rigidity of the silica cage since the isomerization in DODCI is very sensitive to the environment viscosity and requires an increase of the molecular length. The presence of the silica rigid cage affects also the relaxation processes. The time decay of theN-isomer photoluminescence 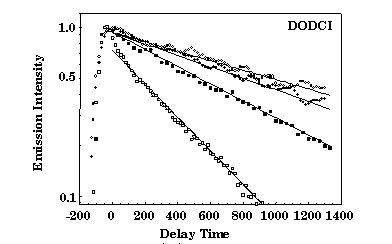 detected at 650 nm was measured with a frequency-mixing technique in a 10-4M methanol solution and in solid samples at three different densification stage. In the case of methanol solution, concentration effects are responsible for the measured value of the decay time t = 450 ps, considerably lower than the expected value of t = 860 ps. At the same concentration of 10-4M, the decay time in as-prepared silica-gel samples recovers the low-concentration value of t = 860 ps, suggesting that the entrapment and separation of the dye molecules quench the intermolecular nonradiative relaxation processes. Moreover, the decay time increases further to t = 1280 ps and t = 1560 ps for samples densified at 80 and 95 ° C, respectively. This indicates that also the intramolecular relaxation pathways are increasingly quenched by the shrinkage of the silica cage.
detected at 650 nm was measured with a frequency-mixing technique in a 10-4M methanol solution and in solid samples at three different densification stage. In the case of methanol solution, concentration effects are responsible for the measured value of the decay time t = 450 ps, considerably lower than the expected value of t = 860 ps. At the same concentration of 10-4M, the decay time in as-prepared silica-gel samples recovers the low-concentration value of t = 860 ps, suggesting that the entrapment and separation of the dye molecules quench the intermolecular nonradiative relaxation processes. Moreover, the decay time increases further to t = 1280 ps and t = 1560 ps for samples densified at 80 and 95 ° C, respectively. This indicates that also the intramolecular relaxation pathways are increasingly quenched by the shrinkage of the silica cage.



 Dynamics of photoisomerization from the initial N-isomer to the P-isomer conformation is described satisfactorily with a three valley energy diagram involving an intermediate state. The physical constraint imposed on the molecules by the dimensions of the silica cage after densification affects the electronic structure and increases the energy of the N-isomer excited state. This results in a blu-shift of the absorption and emission bands of about 20 nm with respect to the bands in not-densified samples (thin line) which coincide with those in liquid solution (10-6M solution in methanol, dashed line). It should be noted, however, that in the nondensified samples under consideration, the absorption spectrum differs from that of methanol because of a new absorption band at approximately 490 nm. This new structure was attributed to the interaction between the silanol groups of the silica cage and a fraction of the dye molecules that are trapped in the pores of the glass matrix. Moreover the emission spectra show that, differently from the liquid solution where the P- to N-isomer emission intensity grows with the average intensity of the exciting light (300 mW/cm2, dashed line; 50 W/cm2, dash-dotted line), the photoisomerization process is strongly quenched (full line). This is likely to occur because of the rigidity of the silica cage since the isomerization in DODCI is very sensitive to the environment viscosity and requires an increase of the molecular length. The presence of the silica rigid cage affects also the relaxation processes. The time decay of theN-isomer photoluminescence
Dynamics of photoisomerization from the initial N-isomer to the P-isomer conformation is described satisfactorily with a three valley energy diagram involving an intermediate state. The physical constraint imposed on the molecules by the dimensions of the silica cage after densification affects the electronic structure and increases the energy of the N-isomer excited state. This results in a blu-shift of the absorption and emission bands of about 20 nm with respect to the bands in not-densified samples (thin line) which coincide with those in liquid solution (10-6M solution in methanol, dashed line). It should be noted, however, that in the nondensified samples under consideration, the absorption spectrum differs from that of methanol because of a new absorption band at approximately 490 nm. This new structure was attributed to the interaction between the silanol groups of the silica cage and a fraction of the dye molecules that are trapped in the pores of the glass matrix. Moreover the emission spectra show that, differently from the liquid solution where the P- to N-isomer emission intensity grows with the average intensity of the exciting light (300 mW/cm2, dashed line; 50 W/cm2, dash-dotted line), the photoisomerization process is strongly quenched (full line). This is likely to occur because of the rigidity of the silica cage since the isomerization in DODCI is very sensitive to the environment viscosity and requires an increase of the molecular length. The presence of the silica rigid cage affects also the relaxation processes. The time decay of theN-isomer photoluminescence  detected at 650 nm was measured with a frequency-mixing technique in a 10-4M methanol solution and in solid samples at three different densification stage. In the case of methanol solution, concentration effects are responsible for the measured value of the decay time t = 450 ps, considerably lower than the expected value of t = 860 ps. At the same concentration of 10-4M, the decay time in as-prepared silica-gel samples recovers the low-concentration value of t = 860 ps, suggesting that the entrapment and separation of the dye molecules quench the intermolecular nonradiative relaxation processes. Moreover, the decay time increases further to t = 1280 ps and t = 1560 ps for samples densified at 80 and 95 ° C, respectively. This indicates that also the intramolecular relaxation pathways are increasingly quenched by the shrinkage of the silica cage.
detected at 650 nm was measured with a frequency-mixing technique in a 10-4M methanol solution and in solid samples at three different densification stage. In the case of methanol solution, concentration effects are responsible for the measured value of the decay time t = 450 ps, considerably lower than the expected value of t = 860 ps. At the same concentration of 10-4M, the decay time in as-prepared silica-gel samples recovers the low-concentration value of t = 860 ps, suggesting that the entrapment and separation of the dye molecules quench the intermolecular nonradiative relaxation processes. Moreover, the decay time increases further to t = 1280 ps and t = 1560 ps for samples densified at 80 and 95 ° C, respectively. This indicates that also the intramolecular relaxation pathways are increasingly quenched by the shrinkage of the silica cage.